Abstract
Introduction
GvHD is a threat to the wellbeing and survival of patients following hematopoietic stem cell transplantation (HSCT). In-vivo and ex-vivo T cell depletion are the most effective methods for GvHD prevention, but attendant risks of infection, graft failure and leukemic recurrence offset the advantages of these manipulations. Less extensive T cell depletion leaves many patients at risk for the ravages of treatment-resistant GvHD. Alternative approaches to eliminate GvHD-causing cells are needed. Fas ligand (FasL), a member of the tumor necrosis factor (TNF) family, can selectively induce apoptosis of mature T cells while sparing stem and progenitor cells. We have developed a novel FasL-mediated selection process which eliminates mature T cell subsets in hematopoietic stem cell grafts and effectively prevents GvHD in pre-clinical models without impairing engraftment and immune system recovery. We report here data from ex-vivo and in-vivo studies supporting the use of FasL-mediated depletion of mature T-cells, a process we have named ApoGraft, as a novel effective method for GvHD prophylaxis in the context of allogeneic HSCT.
Methods:
Aliquots of mobilized peripheral blood cells (MPBC) were collected from 104 consenting healthy donors and were used ex-vivo for optimization of the ApoGraft manufacturing process. ApoGraft is manufactured under GMP guidelines, and includes two washing steps, a 2 hr incubation of the graft at 37oC with human recombinant different concentrations of FasL (MegaFasL, Adipogen), and 2 additional washing steps to remove unbound FasL. Aliquots from 25 MPBC apheresis that had been subjected to the complete ApoGraft process were analyzed ex-vivo for cell yield, viability and cellular constitution by FACS and for clonogenicity potential by colony forming unit (CFU) assays. Additionally, engraftment and GvHD prevention of ApoGraft were evaluated with 7 aliquots of MPBC apheresis in NOD-SCID IL2-Rgamma-null (NSG) mice (n=349). Here we report results from representative studies were unfractionated ApoGraft (2.5x10^6 cells) or CD34+ purified cells (1.0x10^5 cells) from ApoGraft were transplanted into irradiated (2-2.75Gy) NSG mice. The mice were monitored clinically for GVHD occurrence post transplantation; engraftment and differentiation were evaluated using FACS analysis.
Results:
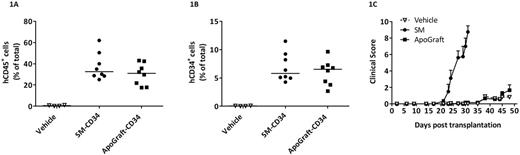
Cellular viability was 98% (±1%) in the ApoGraft samples analyzed. Comparison of ApoGraft and Starting Material (SM) showed a mean 19.9% (±4.3%) reduction of total CD3+ T cells; early apoptosis of CD3+ cell increased by 5-fold as compared to Starting Material. Importantly, the percentage of viable CD34+ stem and progenitor cells was not affected by ApoGraft processing. Proliferation and differentiation of hematopoietic progenitors in colony forming unit (CFU) assay was not affected. In NSG mice infused with unfractionated ApoGraft or CD34+ cells purified from it, chimerism level and differentiation were similar to those of mice infused with control MPBC (figure 1A-1B). Most importantly, ApoGraft infused mice had lower GvHD clinical score and prolonged survival as compared to mice infused with control MPBC (maximal score 1.7±0.7 vs. 8.8±0.8) (Figure 1C).
Conclusion:
ApoGraft is an apoptosis-based selection process that eliminates mature T cells from stem cells grafts, while preserving CD34+ cells quantity, quality and functionality. In our preclinical murine model, ApoGraft reduced GvHD without impairing engraftment. Ongoing animal studies are evaluating the graft vs. leukemic capabilities of ApoGraft and the preservation of anti-viral immunity after ApoGraft processing. Additionally, ApoGraft is currently being evaluated in a Phase I/IIa study (NCT02828878) in subjects with hematological malignancies undergoing matched related allogeneic HSCT.
Zuckerman: BioSight Ltd.: Consultancy. Yarkoni: Cellect Biotechnology Ltd.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Nechushtan: Cellect Biotechnology Ltd.: Employment. Rodionov: Cellect Biotechnology Ltd.: Employment. Gez: Cellect Biotechnology Ltd.: Employment. Rosenzwaig: Cellect Biotechnology Ltd.: Employment. Rodin: Cellect Biotechnology Ltd.: Employment. Pereg: Cellect Biotechnology Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal